
Massive accumulation of L-canavanine, the L-2-amino-4-(guanidinooxy)butyric acid structural analog of L-arginine, occurs in many legumes. Accumulation of large amounts of this nonprotein amino acid results in large part from canavanine protective efficacy; it forms an effective chemical barrier to predation, disease, and even competition with other plants. Diversion of metabolic resources for the synthesis and storage of appreciable canavanine does not place an inordinate burden on the plant. Catabolism of this nonprotein amino acid provides respiratory carbon, generates essential primary metabolites, and ammoniacal nitrogen for the developing plant.
The nonprotein amino acid L-canavanine, the L-2-amino-4(guanidinooxy)butyric acid structural L-arginine analog, is found in at least 1500 members of the Lotoideae, a major subdivision of the Faabaceae; its occurrence is limited strictly to this group of angiosperms ( 1 ).

Canavanine storage in the seeds of such plants can account for more than 10% of the seed dry matter and 90% of the seed nitrogen allocated to free amino acids.
I have striven to determine why so much canavanine is sequestered by certain legumes. A significant part of the reason is provided by investigations establishing that this nonprotein amino acid can be a highly effective protective allelochemical that can exhibit potent antimetabolic activity toward a wide range of pests, competitors, and/or predators of canavanine-storing plants (l0). The purpose of my review, however, is not to illuminate the allelochemical properties and functions of canavanine, but rather to focus on higher plant metabolism of L-canavanine and its metabolic derivative, L-canaline. Analysis of canavanine and canaline metabolism allows one to address the question of whether or not the sequestration of so much nitrogen in canavanine is metabolically efficient or rather a wasteful process that can be rationalized in terms of the benefit accrued by canavanine's protective efficacy. Recent studies of the jack bean, Canavalia ensiformis [Fabaceae], have contributed significantly to the answer of this question.
CANALINE FORMATION
Arginase (EC 3.5.3.1), distributed widely perhaps universally in canavanine-accumulating legumes, catalyzes the hydrolytic cleavage of L-canavanine to L-canaline and urea.
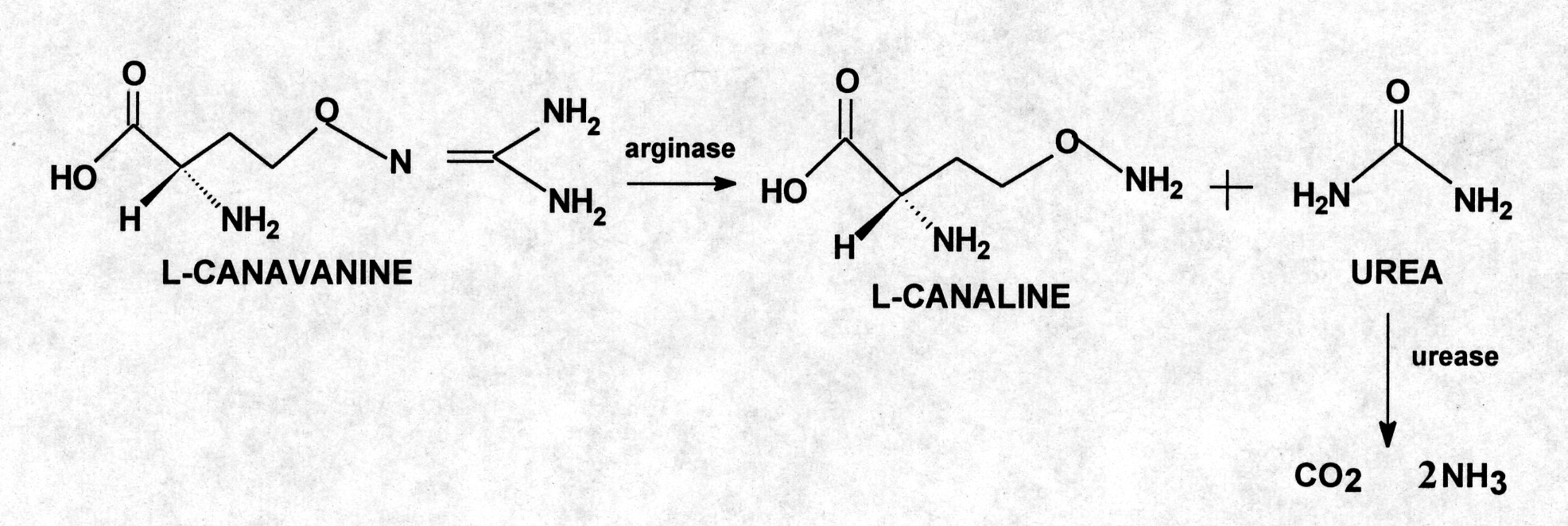
Urease (EC 3.5.1.5) also appears to be distributed universally in canavanine-containing plants and is responsible for converting urea to carbon dioxide and ammonia. A positive correlation exists between seed canavanine content and seed urease activity. The sequential actions of arginase and urease on canavanine, which is the predominate path for canavanine catabolism in canavanine-containing plants (11), yield ultimately canaline as well as C02 and ammonia. Thus, the combined action of arginase and urease in canavanine-producing legumes ensures that one-half of the stored nitrogen of canavanine is released as ammonia. This ammonia can then be utilized by the nitrogen machinery of the developing plant. The production of ammoniacal nitrogen from canavanine has an undesirable consequence—formation of canaline, a potentially deleterious ornithine analog.
Canaline is a poisonous natural product that can produce severe developmental aberrations in the pupal and adult insect, prevent larval-pupal metamorphosis, and create massive pupal deformities, as demonstrated in the tobacco hornworm, Manduca sexta [Sphingidae], which is sensitive to this antimetabolite (4). In addition, canaline induces neurotoxic effects in the adult moth.
The toxic action of canaline results primarily from its facile reaction with the carbonyl group of aldehydes or certain organic acids to form a stable oxime. For example, canaline reacts chemically with 2-oxoglutaric acid to form stoichiometrically a canaline-2-oxoglutaric acid oxime (3). Canaline also reacts nonenzymatically to form oximes with pyruvic acid and oxaloacetic acid; these reactions can deplete tricarboxylic acid cycle compounds. Canaline also forms an oxime with pyridoxal phosphate; production of the canaline-pyridoxal phosphate oxime inactivates rapidly vitamin B6-containing enzymes (9,13). Canaline's powerful inhibition of pyridoxal phosphate-containing enzymes, including many decarboxylases and aminotransferases, is documented (9,13).
CANALINE AS A PROTECTIVE ALLELOCHEMICAL
In principle, canaline should be an efficacious, protective allelochemical for canavanine-storing legumes because canaline can elicit antimetabolic and insecticidal effects as pronounced as those of canavanine. Yet the canaline content of Canavalia ensiformis is meager. Ontogenetic analyses of canaline in the root, shoot, cotyledons, and leaves of C. ensiformis, conducted over a 24-d period, reveal typically <0.1 micromole canaline per 100 mg dry matter (16). Inatomi et al. (6) reported the isolation of only 200 mg of canaline from 50 kg of Astragalus sinicus seeds, about 4 ppm. Because essential metabolites are scavenged and critical enzymes inactivated, canaline is sufficiently deleterious to the plant itself, protective regulatory processes have evolved that ensure the level of canaline remains low.
CANALINE DETOXIFICATION
Canaline's intrinsic toxicity, its appreciable nitrogen content, and low natural abundance leads one to expect that canaline-producing legumes have evolved mechanisms for its detoxification and the utilization of its stored nitrogen. An important canaline detoxification mechanism is disclosed by studies of C. ensiformis that revealed that canaline reacts rapidly with glyoxylate to form a canaline-glyoxylate oxime (Fig. 1, reaction c) (14).
The primary leaves of C. ensiformis contain an enzyme able to mediate the reductive cleavage of the oxygen-nitrogen bond of the aminooxy function of canaline. This protein uses NADPH to mediate an irreversible cleavage of canaline to generate homoserine and NH3 (Fig. 1, reaction b). The irreversible nature of the reaction is not unexpected for it prevents deleterious canaline formation from homoserine. This pathway for homoserine production from canavanine through canaline is unique to higher plants. The evolution of such a mechanism enables higher plants to release additional nitrogen from canavanine as ammonia rather than as guanidine or hydroxyguanidine. There is not any evidence that higher plants are able to use these guanidine compounds; thus, their formation would waste nitrogen.
Canavanine can be converted to homoserine in certain bacteria but only by direct reduction to form guanidine (8) or hydrolysis to yield hydroxyguanidine (7). The tobacco budworm, Heliothis virescens [Noctuidae], has a marked ability to detoxify canavanine (2); our research indicates that this canavanine-resistant insect achieves this by reduction of canavanine to homoserine and guanidine. Thus, the available evidence indicates that neither bacteria nor H. virescens convert canavanine to canaline prior to homoserine formation; rather, homoserine forms directly from canavanine.
Sugii et al. (16) reported the isolation of 3-isoxazolidone from the jack bean. These workers proposed that canaline is deaminated to form its corresponding 2-oxo derivative and then decarboxylated to an aldehyde that is converted finally to 3-isoxazolidone (Fig. 1, reaction d). The latter compound can be converted to 3-oxoproprionic acid; this reaction would release nitrogen from canaline as ammonia ( 16).
Administration of L-[U-14C]canaline to 9 d old C. ensiformis plants produces a number of radiolabeled degradation compounds; homoserine, phosphohomoserine, canaline-glyoxylate, Iysine, and canavanine ( 12). Analysis of the metabolic fate of canaline, and thereby essentially canavanine, reveals the extent to which these amino acids support the primary metabolic reactions of the plant (Fig. 1, reactions e and g). About one-third of the radiolabeled carbon isolated from the jack bean plant is found in acetone-soluble materials. The radiocarbon in this fraction decreases rapidly with time, with a concomitant rise in the respiratory loss of carbon as C02. These data suggest that much of the aliphatic carbon skeleton of canavanine or canaline is respired to C02. About 60% of the radiolabeled carbon found in the charged substances is homoserine. In addition, radiolabeled phosphohomoserine is produced. This is a significant finding as this phosphorylated amino acid is the precursor for cystathionine and homocysteine in higher plants (5). These sulfur-containing amino acids, in turn, are converted to cysteine and methionine as well as S-adenosylmethionine, respectively. Moreover, homoserine derived from canaline supports the biosynthesis of Ivsine; this observation has been confirmed by independent feeding studies involving L-[U-I4C]homoserine (11) (Fig. 1, reaction e).
There is a very small but significant synthesis of canavanine from canaline. This synthesis probably occurs by carbamylation of canaline to form O-ureidohomoserine and conversion of the latter to canavaninosuccinic acid prior to canavanine formation. It is not possible to prove the in viva existence of these metabolic reactions constituting a pathway analogous to the mammalian Krebs-Henseleit ornithine-urea cycle because it has not been possible to isolate detectable levels of O-ureidohomoserine nor canavaninosuccinic acid from the jack bean plant. On the other hand, one can isolate enzymes from jack bean capable of producing O-ureidohomoserine from canaline and canavanine from canavaninosuccinic acid. In any event, the above sequential reaction from canaline to canavanine is only of limited physiological significance in the biosynthesis of canavanine. The recent discovery of the deleterious properties of canaline and its low natural abundance are not consistent with a role for canaline as a significant canavanine precursor. The biochemical basis for the formation of substantial amounts of canavanine remains unknown.
Demonstration of canavanine's contribution to the nitrogen metabolism of the young jack bean plant has been made possible by the synthesis of L-[guanidinooxy-N3,5N]canavanine (15). Because a heavy nitrogen is in the terminal guanidinooxy moiety, utilization of the isotope reveals the metabolic fate of canavanine’s nitrogens that are converted, via the action of arginase, initially to [15N]urea and canaline.
Mass spectrometric analysis of the labeling pattern in such 15N-treated jack bean plant reveals marked labeling of glutamic and aspartic acids and/or their corresponding amides (our mass spectrometer technique does not differentiate between these amino acids and their amide derivative). Histidine, isoleucine, serine, valine, arginine, and especially alanine also are labeled significantly (15).
L-[Guanidinooxy-N1,15N]canavanine has also been prepared (15). The action of arginase on L-[guanidinooxy-N1,15N]canavanine converts this compound initially to [15N]canaline and urea prior to further utilization. A similar 15nitrogen-labeling pattern results from the use of both forms of 15N-labeled canavanine, with one exception. L-[Guanidinooxy-N1,15N]canavanine produces nearly three times as much alanine as does L-[guanidinooxy-N3,15N]canavanine, primarily at the expense of glutamate/glutamine. Thus, the pattern of utilization of the nitrogen of canavanine first diverted to urea is different in some presently unknown way from that of nitrogen diverted to canaline (15). All of the available evidence attests to the ability of canavanine to support primary metabolic reactions of the developing plant. This ability explains why a legume can accumulate such massive levels of canavanine. The carbon skeleton of canavanine is catabolized for energy-yielding respiratory reactions, used to produce primary metabolites, and its nitrogen is assimilated into a plethora of reactions creating important plant metabolites. A plant's ability to use canavanine for primary metabolism allows one to understand that the accumulation of canavanine to serve as a protective allelochemical does not place an inordinate metabolic burden on the plant.
LITERATURE CITED
1. Bell EA, Lakey JA, Pohill RM (1978) Systemic significance of canavanine in the Papilionideae (Fabiodeae). Biochem Sys Ecol 6: 201-212
2. Berge MA, Rosenthal GA, Dahiman DL (1986) Tobacco budworm, Heliothis virescens (Noctuidae) resistance to L-canavanine, a protective allelochemical. Pestic Biochem Physiol 25: 319-326
3. Cooper AJL (1984) Oxime formation between 2-keto acids and L-canaline. Arch Biochem Biophys 233: 603-610
4. Dahlman DL, Rosenthal GA (1975) Non-protein amino acidinsect interactions. I. Growth effects and symptomologv of Lcanavanine consumption by the tobacco hornworm, Manduca sexta (L.). Comp Biochem Physiol 51A: 33-36
5. Giovanelli J. Mudd SH, Datko AH (1974) Homoserine esterification in green plants. Plant Physiol 54: 725-736
6. Inatomi H. Inugai F. Murakami T (1968) Isolierung und Identifizierung von Canalin in unreifen Samen von Astragulus sinicus L. Chem Pharm Bull 16: 2521
7. Kalyankar GD, Ikawa M, Snell EE (1958) The enzymatic cleavage of canavanine to homoserine and hydroxyguanidine. J Biol Chem 233: 1175-1178
8. Kihara H. Prescott JM, Sncil EE (1957) The bacterial cleavage of canavanine to homoserine and guanidine. J Biol Chem 226: 497-503
9. Rahiala E-L, Kekomaki M, Janne J. Raina A, Ralha NCR (1970) Inhibition of pyridoxal enzymes by L-canaline. Biochim Biophys Acta 227: 337-343
10. Rosenthal GA (1982) Plant Nonprotein Amino Acids: Biological. Biochemical and Toxicological Properties. Academic Press. New York, p 273
11. Rosenthal GA (1982) L-canavanine metabolism in jack bean, Canavalia ensiformis (L.) DC. (Leguminosae). Plant Physiol 69: 1066- 1069
12. Rosenthal GA, Berge MA (1989) Catabolism of L-canavanine and L-canaline in the jack bean, Canavalia ensiformis (L.) DC. J Food Agr Chem 37: 591-595
13. Rosenthal GA, Dahiman DL (1990) Interaction of L-canaline with ornithine aminotransferase of the tobacco hornworm. Manduca sexta. J Biol Chem 265: 868-873
14. Rosenthal GA, Berge M, Bleiler J (1989) A novel higher plant mechanism for the detoxification of L-canaline. Biochem Syst Ecol 17: 203-206
15. Rosenthal GA, Berge M, Ozinskas A, Hughes CH (1988) Ability of L-canavanine to support nitrogen metabolism in the jack bean, Canavalia ensiformis. J Agric Food Chem 36: 11591163
16. Sugli M, Miura H. Nagata K (1981) 3-lsoxazolidone from jack bean seedlings. Phytochemistry 20: 451 -453