L-CANAVANINE
WEB
SITE
GERALD A. ROSENTHAL
garose@az.rmci.net
7064 East Eagle Feather Road
Scottsdale, AZ 85262-7112
INTRODUCTION
Higher
plants synthesize and store a large number of natural products that are
part of their repertoire of essential or primary
metabolites,
such as glucose or adenosine 5'-triphosphate; these compounds are distributed
uniformly throughout the Plant Kingdom. In contrast, the secondary
metabolites
of
higher plants do not occur universally-i.e. they are found in certain plants
and not in others. These secondary metabolites are none-the-less
important because they contribute decisively to the distinct chemical properties
of a given plant. Many of the
individual, defining
chemical
characteristics of a given higher plant reflect their natural variation
in the occurrence and concentration of their secondary metabolites.
Numerous toxic substances,
including a number of nonprotein amino acids, are part of this idiosyncratic
assemblage of secondary metabolites; they constitute a vital group of compounds
which function in plant defense against herbivores, predators and pathogens.
These protective compounds are allelochemicals that
play
a critical role in organismic interactions involving plants; yet, only
in limited instances do we understand their mode of action at the biochemical
level.
This deficiency has motivated
my career-long interest in L-canavanine
a nonprotein amino acid synthesized by leguminous plants that is a potent
L-arginine
antimetabolite. I wanted to understand the raison d'etre for this
toxic metabolite and how it functioned to provide effective protection
to the plant-particularly against insects.
Want to learn more about the remarkable
ability of higher plants to mount a formidable chemical defense against
their most formidable enemy-the insects?
L-Canavanine is one of approximately
600 naturally-occurring, nonprotein amino acids that have been isolated
and chemically characterized. This arginine antimetabolite is synthesized
only by leguminous plants (such as clover, alfalfa, trefoils, and Lespedeza)
which are members of a large assemblage of higher, vascular plants collectively
grouped into the Fabaceae- a major family of higher plants..

Not all leguminous plants
produce canavanine, but it does occur in hundreds of legumes. While canavanine
can be found in all tissues of the living plant, even the petals of the
flower, it is stored primarily in the seeds where it serves to protect
this organ from many potential consumers-particularly insects. With its
high nitrogen to carbon ratio, canavanine is also an effective
nitrogen-storing metabolite. As such, it functions to provide nitrogen
for the developing embryo.
IS
DIETARY CANAVANINE HARMFUL TO HUMANS?
 DO
YOU WANT TO LEARN MORE ABOUT SYSTEMIC LUPUS ERYTHEMATOSUS?
DO
YOU WANT TO LEARN MORE ABOUT SYSTEMIC LUPUS ERYTHEMATOSUS?
 DO
YOU WANT TO LEARN MORE ABOUT THE NATURAL ABUNDANCE OF CANAVANINE IN ALFALFA
SEEDS AND SPROUTS?
DO
YOU WANT TO LEARN MORE ABOUT THE NATURAL ABUNDANCE OF CANAVANINE IN ALFALFA
SEEDS AND SPROUTS?
CANAVANINE
CHEMISTRY
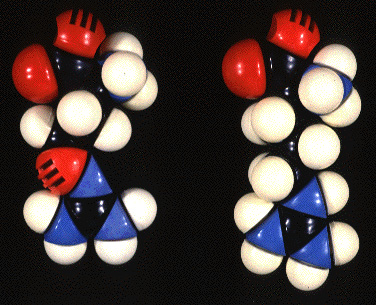 L-CANAVANINE
L-ARGININE
L-CANAVANINE
L-ARGININE
Examination of these
two amino acids reveals some interesting similarities and differences.
Overall, the molecules are remarkably similar, but the terminal methylene
group linked to the guanidino moiety of arginine is replaced by
oxygen (depicted in red) in canavanine to
create a novel functionality-a guanidinooxygroup.
The pKa of the guanidino group of arginine is 12.48 as compared
to 7.04 for the guanidinooxy moiety.This property causes canavanine to
be far more acidic an amino acid than is arginine.Under
physiological conditions, the guanidino group of arginine is virtually
fully protonated and positively charged. Under
such conditions, many of the canavanine molecules have a deprotonated guanidinooxy
group and therefore are not charged.
CANAVANINE-CONTAINING
PROTEINS
Because of its structural
similarity to arginine, canavanine is a substrate for the enzyme  ARGININYL-tRNA
SYNTHETASE. This protein is responsible for placing arginine
into the growing polypeptide chain. Incorporation of canavanine into a
protein in place of arginine results in a structurally aberrant, canavanine-containing
macromolecule. Replacement of arginyl residues within
a protein by canavanine can result in the loss of critically positioned
charged groups within the macromolecule. This can adversely affect R-group
interactions that are essential to creating the unique three dimensional
conformation of a given protein. In
fact, canavanyl proteins suffer diminished activity and lost functionality
which contributes to canavanine's antimetabolic properties.
ARGININYL-tRNA
SYNTHETASE. This protein is responsible for placing arginine
into the growing polypeptide chain. Incorporation of canavanine into a
protein in place of arginine results in a structurally aberrant, canavanine-containing
macromolecule. Replacement of arginyl residues within
a protein by canavanine can result in the loss of critically positioned
charged groups within the macromolecule. This can adversely affect R-group
interactions that are essential to creating the unique three dimensional
conformation of a given protein. In
fact, canavanyl proteins suffer diminished activity and lost functionality
which contributes to canavanine's antimetabolic properties.
INSECT-BASED
CANAVANINE STUDIES
 Investigations
of the neotropic bruchid beetle,
Investigations
of the neotropic bruchid beetle, Caryedes
brasiliensis,[Bruchidae] the
sole predator of the canavanine-laden seeds of the neotropical legume,
Dioclea megacarpa
[Fabaceae].
Caryedes
brasiliensis,[Bruchidae] the
sole predator of the canavanine-laden seeds of the neotropical legume,
Dioclea megacarpa
[Fabaceae].
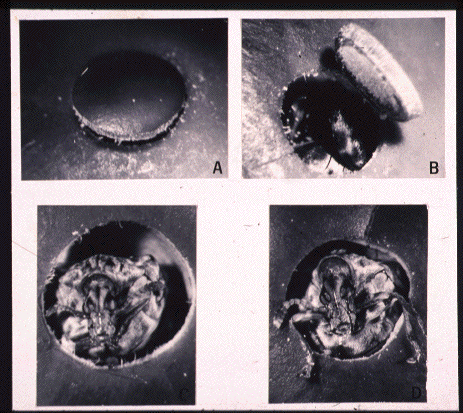
Learn about this fascinating
seed predator seen emerging as an adult from the seed of Dioclea megacarpa.
The
female oviposits her eggs on the fruit of this neotropical legume. The
newly emerged larvae penetrate the fruit and develop within the seeds which
can store as much as 13% canavanine by dry weight.
 Elucidating the biochemical basis for the remarkable tolerance of the tobacco
budworm,
Elucidating the biochemical basis for the remarkable tolerance of the tobacco
budworm,  Heliothis
virescens [Noctuidae] to canavanine.
Heliothis
virescens [Noctuidae] to canavanine.
 Probing the
Probing the BIOCHEMICAL
BASIS FOR CANAVANINE'S ANTIMETABOLIC PROPERTIES IN INSECTS.
BIOCHEMICAL
BASIS FOR CANAVANINE'S ANTIMETABOLIC PROPERTIES IN INSECTS.
PLANT-BASED
CANAVANINE STUDIES
Investigations
of  CANAVANINE CATABOLISM IN THE JACK BEAN,Canavalia ensiformis
[Fabaceae] have provided an in-depth picture of how the nitrogen atoms
of the guanidinooxy group of canavanine are mobilized for subsequent use
by the developing plant.
CANAVANINE CATABOLISM IN THE JACK BEAN,Canavalia ensiformis
[Fabaceae] have provided an in-depth picture of how the nitrogen atoms
of the guanidinooxy group of canavanine are mobilized for subsequent use
by the developing plant.
CANAVANINE
AS A CHEMOTHERAPEUTIC AGENT
The  ANTINEOPLASTIC
ACTIVITY OF THIS NONPROTEIN AMINO ACID has been demonstrated by in
vivo and in vitro studies. Canavanine effects on human pancreatic
cell lines have been studied.
ANTINEOPLASTIC
ACTIVITY OF THIS NONPROTEIN AMINO ACID has been demonstrated by in
vivo and in vitro studies. Canavanine effects on human pancreatic
cell lines have been studied.
L-CANALINE
Enzymatic
hydrolysis of L-canavanine
by arginase (EC 3.1.2.5) yields urea and a novel nonprotein amino acid,
L-canaline
that bears structurally analogy to L-ornithine.
L-Canaline,
L-2-amino-4-(aminooxy)butyric acid, is unique in being
the only naturally occurring amino acid possessing an aminooxy moiety:
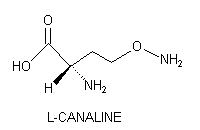 Canaline
reacts vigorously with the pyridoxal phosphate moiety of vitamin B6-containing
enzymes to form a covalently-bound oxime that inactivates, often irreversibly,
the enzyme. As such it is a powerful antimetabolite that is formed in any
canavanine-storing legume since arginase is distributed universally in
this family of higher plants. Recently, this natural product was shown
to exhibit significant antineoplastic activity against MIAPaCa-2, a human
pancreatic cancer cell line.
Canaline
reacts vigorously with the pyridoxal phosphate moiety of vitamin B6-containing
enzymes to form a covalently-bound oxime that inactivates, often irreversibly,
the enzyme. As such it is a powerful antimetabolite that is formed in any
canavanine-storing legume since arginase is distributed universally in
this family of higher plants. Recently, this natural product was shown
to exhibit significant antineoplastic activity against MIAPaCa-2, a human
pancreatic cancer cell line.
 THE
BIOCHEMICAL AND ANTINEOPLASTIC PROPERTIES OF CANALINE
THE
BIOCHEMICAL AND ANTINEOPLASTIC PROPERTIES OF CANALINE
 Last
revision: 1, April 1999
Much of the research documented on this web site was made possible by
a series of research grants provided by the National Science Foundation
Last
revision: 1, April 1999
Much of the research documented on this web site was made possible by
a series of research grants provided by the National Science Foundation
 Your comments and inquiries are solicited: garose@az.rmci.net
Your comments and inquiries are solicited: garose@az.rmci.net



![]() ARGININYL-tRNA
SYNTHETASE. This protein is responsible for placing arginine
into the growing polypeptide chain. Incorporation of canavanine into a
protein in place of arginine results in a structurally aberrant, canavanine-containing
macromolecule. Replacement of arginyl residues within
a protein by canavanine can result in the loss of critically positioned
charged groups within the macromolecule. This can adversely affect R-group
interactions that are essential to creating the unique three dimensional
conformation of a given protein. In
fact, canavanyl proteins suffer diminished activity and lost functionality
which contributes to canavanine's antimetabolic properties.
ARGININYL-tRNA
SYNTHETASE. This protein is responsible for placing arginine
into the growing polypeptide chain. Incorporation of canavanine into a
protein in place of arginine results in a structurally aberrant, canavanine-containing
macromolecule. Replacement of arginyl residues within
a protein by canavanine can result in the loss of critically positioned
charged groups within the macromolecule. This can adversely affect R-group
interactions that are essential to creating the unique three dimensional
conformation of a given protein. In
fact, canavanyl proteins suffer diminished activity and lost functionality
which contributes to canavanine's antimetabolic properties.
![]() Investigations
of the neotropic bruchid beetle,
Investigations
of the neotropic bruchid beetle,![]() Caryedes
brasiliensis,[Bruchidae] the
sole predator of the canavanine-laden seeds of the neotropical legume,
Dioclea megacarpa
[Fabaceae].
Caryedes
brasiliensis,[Bruchidae] the
sole predator of the canavanine-laden seeds of the neotropical legume,
Dioclea megacarpa
[Fabaceae].

![]() Elucidating the biochemical basis for the remarkable tolerance of the tobacco
budworm,
Elucidating the biochemical basis for the remarkable tolerance of the tobacco
budworm, ![]() Heliothis
virescens [Noctuidae] to canavanine.
Heliothis
virescens [Noctuidae] to canavanine.
![]() Probing the
Probing the![]() BIOCHEMICAL
BASIS FOR CANAVANINE'S ANTIMETABOLIC PROPERTIES IN INSECTS.
BIOCHEMICAL
BASIS FOR CANAVANINE'S ANTIMETABOLIC PROPERTIES IN INSECTS.
![]() CANAVANINE CATABOLISM IN THE JACK BEAN,Canavalia ensiformis
[Fabaceae] have provided an in-depth picture of how the nitrogen atoms
of the guanidinooxy group of canavanine are mobilized for subsequent use
by the developing plant.
CANAVANINE CATABOLISM IN THE JACK BEAN,Canavalia ensiformis
[Fabaceae] have provided an in-depth picture of how the nitrogen atoms
of the guanidinooxy group of canavanine are mobilized for subsequent use
by the developing plant.

