TSNA storage sub-committee
Objective:
To determine TSNA change in burley and dark air-cured tobacco leaf from end-of-curing until initial processing (threshing and redrying).
To achieve this objective samples would be normally taken at stripping, if stalk-cured, if stripping occurs soon after curing is complete. Importantly a representative sample must be taken from a “bulk” of tobacco at different times. For example this may be (1) at stripping, (2) at delivery to the receiving station or warehouse, (3) just prior to initial processing, and (4) the end of the initial processing. (Samples may lose their identity at this point and this sample will not be useful.) This example assumes that there are a few weeks between the first 3 events and the last is to determine what is going into the package for storage. A sample should be taken every time there is significant storage time or movement of a “lot” of tobacco. The “lot” of tobacco would be a package put together by the grower of sufficient size that it could be identified until threshing/redrying.
If a large quantity of tobacco is taken down from the curing barn when it is in case (order), the tobacco stick removed and the tobacco covered with plastic to store during stripping, this could be a time for TSNA formation. This is a variable that someone may be able to evaluate by taking samples as the tobacco is removed from the sticks and soon after it has been put into a package.
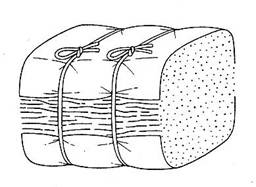
If this package is a bale the best sampling procedure would be to take a core sample of at least 50g. The core sample should be taken from the midpoint of the bale down through several leaves not along the plane of a few leaves. If oriented leaf the sampling would be as shown. The core sample should be taken from the midpoint of the bale down through several leaves not along the plane of a few leaves. If oriented leaf the sampling would be as shown. Our coring device is about 18 inches (45 cm) long with a 1 inch (2.5 cm) opening and is attached to an electric drill. A wooden dowel is used to push the tobacco from the probe.
| ↓ |

If the package is loose leaf, leaves or half-leaves from several different plants would be required to get a representative sample. These should be from the same general stalk position. Getting a representative sample from the package is most significant for interpretation of data obtained.
Sample handling prior to TSNA measurement will depend upon when and where the samples will be analyzed relative to their generation. If the measurements are to be performed locally, the samples must be placed in paper bags and put into dehumidified or air-conditioned space to dry with the maximum temperature 35C/95F. Samples will dry to 8-10% moisture in a few days. If available, freeze-drying the samples would be recommended. Once dry, samples may be sent to a laboratory for analysis. If the dry samples are going to be stored in the dry condition they should be frozen at -20C (household freezer). They may be powdered prior to freezing or just prior to analysis. Midrib/stem should be separated from the lamina prior to grinding. Only the lamina will be chemically analyzed.
TSNA and alkaloids should be determined by the procedures suggested by Tobacco Science Research Conference analytical committee and CORESTA (Morgan et al. 2004 and Chen et al. 2005) . If possible, total nitrogen, nitrate and nitrite should be determined.
If possible, nearly essential, data loggers for temperature and relative humidity should be placed in the storage spaces of the tobacco packages at each step in the handling process. Also, many of these data loggers have probes that could be inserted into the tobacco package to obtain temperatures inside the package.
The above is the minimum needed to obtain data from growers in many locations to meet the objective.
![]() If
an organization has the capacity to conduct more controlled experiments the
following variables might be included.
If
an organization has the capacity to conduct more controlled experiments the
following variables might be included.
1. Burley air-cured seed type (probably not an issue for dark air-cured)
A. Low Converter (LC)
B. Non-Screened Seed Lots
2. Location(s)
To include growers with sufficient production to produce different size package
3. Timing
Utilize large volume producers to take advantage of early stripping or double barning (curing two harvests in one barn in one curing season). This then may include a sample at take-down and storage on the stalk prior to stripping plus a sample based upon method used to get leaf in case/order for bulking prior to stripping.
4. Packaging
A. Traditional Farmer Bales (~ 75 lbs. / 34 kg.), oriented leaf
B. Large Bales (~ 550 lbs. / 250 kg.), non-oriented leaf
(42” x 42” x 40”) / (~ 1 m3)
5. Stalk Positions
6. Bale Moisture
A. Within current receiving station limits (< 24%) but to include different moisture content going into the bales
B. Data Loggers (RH/Temp) for each location of storage
7. Bale Density
Range of low density versus high density packaging, ie different pressure on the baler
8. Bale Storage
A. Storage facility type
B. Stack height and stack density
Chen, P.X., N. Qian, H.R. Burton, and S.C. Moldoveanu. 2005. Analysis of Minor Alkaloids in Tobacco: A Collaborative Study. Beiträge Tabakforschung 21:369-379.
Morgan, W.T., J.B. Reece, C.H. Risner, C.H. Bennett, C.H. Midgett, K.S. Johnson, and H.R. Burton. 2004. A Collaborative Study for the Determination of
Tobacco Specific Nitrosamines in Tobacco. Beiträge Tabakforschung 21: 192-203.