PLS
622: Plant Physiology I
Wednesday, September 13, 2006
Section II: Embryo and Seed
Development:
Lecture
IX: Seed
germination:

yeah…right!
Orthodox seeds undergo maturation
desiccation and some remain on the mother plant for some time in a fruit or
cone. Eventually, however, all seeds are released from the mother plant and
dispersed by the elements or by other living things. It is in the guise of the
seed that the plant is most resistant to external stress. For example, it is
possible to take desiccated orthodox seeds of many species and immerse them in
liquid nitrogen (-196°C), take them
out after one minute or decade, warm them, water them and they complete
germination normally! Their resistant nature permits seeds to withstand periods
of unfavorable environmental perturbation and sustains life until conditions
more favorable to germination and establishment arise. What is germination?
Strictly speaking germination commences when a seed imbibes water and
terminates as soon as some portion of the embryo (usually the radicle) emerges
from the seed covers (e.g. testa, nucellus, perisperm, endosperm). The
inappropriate use of the term to also describe seedling establishment has lead
to considerable confusion and necessitates covering GERMINATION and POST-GERMINATION
events separately for emphasis.
Anhydrobiosis: Mature,
desiccated seeds can have a moisture content of less than 5% by weight and yet
they are alive, they respire, although it is difficult to measure because it
occurs at such a reduced rate. If seeds are capable of completing germination
upon the provision of light, heat, water, and favorable atmospheric conditions,
when desiccated they are said to be quiescent.
If they are incapable of completing germination upon entering favorable
conditions due to some internal impediment to radicle protrusion they are said
to be dormant. We will discuss seed
dormancy and its implications in the next lecture.
Germination
events:
Water
uptake:
Seed water uptake can be divided into three stages (Fig. 1). Imbibition, the first stage, is usually
rapid, commencing with the seed being placed on water where it quickly hydrates
the cells and their constituents. This is followed by the lag phase of water uptake, where there is very little net gain of
water. This is not to say that water is not taken up by the seed. In reality
there is a steady state reached between the amount of water lost by the seed
due to evaporation and that taken up by the seed from its surroundings. In
natural conditions, the amount of water lost by a seed that is placed on the
surface of the ground can actually exceed the amount it takes up from the soil
and limits germination. Finally, the third phase of water uptake is one of
rapid hydration to support the expansion
of the embryo as it completes germination and emerges from the seed (Fig.
1). Due to the impermeability of the testa or other covers, some seeds cannot
take up water freely when placed in contact with it but must wait for some
physical abrasion or partial chemical breakdown to occur prior to imbibing.
The
antagonistic role of
GA is a potent
stimulatory hormone for seed germination. The fact that severe GA deficient
mutants in arabidopsis (ga) and
tomato (gib-1) will not complete
germination without an exogenous supply of GA indicates how crucial this
hormone is to seed germination. The current wisdom is that there is still
sufficient
This is not the whole story. Recent
results from the lab of Peter McCourt have shown that both ethylene and
brassinosteriod act to desensitize the embryo, at least, to
Cellular
events during seed germination…THE original (and persistent) “black box”: The answer to
the children’s song above is still ‘NO!’. But we are making progress. Here is
an overall synopsis of what must occur during the germination event (see also
Figure 1 below). After phase I of water uptake, the cells within the seed must
reconstitute their lipid membranes, repair damage to DNA and protein incurred
during the quiescent phase, replace extensively damaged membranes, resume
transcription, translation, and respiration, and commence instituting the
sensory machinery to enable it to monitor its external environment to determine
if conditions are favorable for establishment or not.
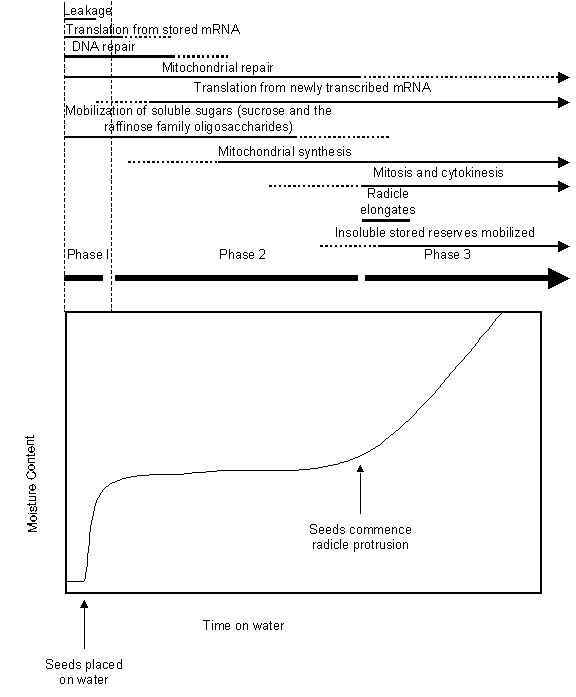
Figure
1:
Time course of water uptake by an orthodox seed depicting the three stages of
imbibition (phase 1), lag (phase 2), and completion of germination (phase 3) as
well as the cellular events thought to occur in each phase (Redrawn and altered
from Bewley 1997, The Plant Cell, 1055-1066).
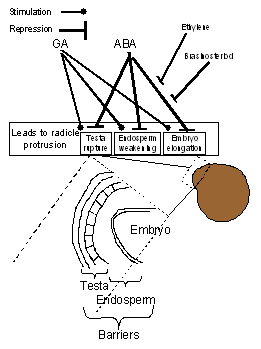
Figure
2:
The antagonistic relationship between GA and
Desiccated
seeds contain an intracellular environment in which the cytoplasmic and
organellar contents are extremely concentrated. High salt concentration, a long
period in the desiccated state, and rapid imbibition, all exacerbates DNA
breakage, loss of membrane integrity, and enzyme inactivation. During
germination, a system capable of orchestrating considerable repair to a variety
of macromolecules is invoked. The natural repair mechanism (NRM) includes both
DNA and protein repair mechanisms. This repair must be effected at a point in
the plant life cycle when energy, reductant, and structural carbon are poorly
available since they are immobilized in storage materials. Additionally, the
mitochondria are not efficient during the early part of germination since their
membranes too have suffered compromise. All of these limitations necessitates
the type of repair, if any, the NRM can conduct.
Despite all of
the activity in repairing damage, and preparing the seed for the completion of
germination, there is not a single visible exterior change discernable between
the live and the dead seed, the desiccated and the imbibed! Even at the
ultrastructural level, very few changes are detectable during seed germination
and those only a few hours prior to the completion of germination. There are,
however, three changes that can be discerned in many seeds that are about to complete germination but have
not yet done so. They are: 1) the differentiation
of the provascular cylinder of
the embryo into xylem and phloem; 2) some very limited mobilization of stored
reserves in the radicle tip and (endospermic seeds only) in the cells of
the micropylar endosperm, and; 3) (again only in endospermic seeds) partial cell wall disassembly of the
cells of the micropylar endosperm ahead of the radicle.
The
differentiation of the provasculature into xylem and phloem is necessary in
order to conduct metabolites from stored reserves located in the cotyledons or
endosperm into the growing radicle and hypocotyl. Additionally, this permits
the embryo to conduct water taken up from the soil immediately upon the
completion of germination to support continued cell expansion throughout the
embryo. Do not forget that, upon successful completion of germination in epigeal (in which the cotyledons are
carried by the hypocotyl above the soil surface as opposed to hypogeal where the cotyledons stay
beneath the soil surface) seedlings, the cotyledons are raised from the surface
of the soil, losing any chance to absorb water by diffusion and therefore are
dependent on functioning vasculature for water supply. The
The
limited hydrolysis of stored reserves in the radicle tip and micropylar
endosperm adds energy and carbon and nitrogen building blocks to the actively
growing radicle prior to and during the commencement of growth. Typically, this
replaces the reserves of sucrose and raffinose family oligosaccharides (RFOs)
that have been exhausted during the first stages of germination (Fig. 1). It
must be emphasized that this mobilization of protein and carbohydrate/lipid is
in a very limited part of the seed and the vast majority of stored reserves of
protein and carbohydrate/lipid remain unmobilized until after the radicle has
protruded from the seed (i.e. is a post-germinative event, see below)!
The
partial disassembly of the cell wall of the region of the endosperm or other
covers opposite the radicle has been studied extensively in endospermic seeds.
Candidate enzymes that hydrolyse the hemicellulosic or pectin components of the
cell wall that have been studied in this regard include endo-b-mannanase
which randomly cleaves polymers of mannose anywhere along the backbone into
shorter fragments, xylo-glucanases that randomly cleave xyloglucan polymers,
expansins that simply disassociate hemicellulose polymers from para-crystalline
cellulose microfibrils without cleavage, xylo-glucan endotransglycosylases that
cleave and then re-polymerize xyloglucan polymers, and polygalacturonases that
cleave single galacturonic acid moieties from the non-reducing ends of polymers
of galacturonic acid (pectin). All of these hydrolases, transglycosylases,
expansins probably play an interactive role in weakening the resistance of the
endosperm or other covers to puncture by the radicle. Hence, it becomes
increasingly easy for the embryo to push through or between the cells of the
covers and emerge. This decrease in resistance to puncture has been demonstrated
for many angiosperm and one conifer seed. Indeed, the requirement of endosperm
weakening to permit radicle protrusion, in endospermic seeds, is one of the few
facets of seed germination that is absolutely known through direct measurements
of resistance to puncture using an instron (instrument capable of measuring
extremely small forces). Additionally, the pleiotropic mutant deficient in
gibberellic acid in both arabidopsis (ga) and tomato (gib-1) does not complete
germination unless the seeds are provided with exogenous gibberellic acid or
the testa and endosperm cap are removed surgically. The endosperm cap of the
tomato gib-1 mutant does not weaken
as the wild type does unless exogenous GA is applied.
Work
on the poor germinating, GA-insensitive mutants sleepy and sneezy has
uncovered a series of 5 proteins (GA-INSENSITIVE (GAI), REPRESSOR OF GA (RGA),
REPRESSOR OF GA-LIKE1 (RGL1), RGL2 and RGL3). These proteins are collectively named
‘DELLA’ proteins because they all possess this highly conserved, pentameric
amino acid combination in their amino-terminus. They are all inhibitors of GA
action, including seed germination. In the presence of the DELLA proteins, GA
fails to stimulate cellular metabolism leading to the completion of
germination. These negative regulators of the GA response (DELLA proteins) must
be actively tagged and degraded. This is accomplished in normal seeds by the
SLEEPY and SNEEZY proteins. SLEEPY and SNEEZY are ‘F-box proteins’. F-box
proteins can attach to target proteins, take them to the poly-ubiquitination
complex and position them so that the ubiquitin ligases of the complex can polyubiquinate
the target protein. This slates the target protein for destruction by the 26S
proteasome. Without SLEEPY and/or SNEEZY the DELLA proteins persist in the
cells of the seed, interfering with the ability of GA to stimulate germination.
Non-germination
may be due simply to a lack of sufficient energy. Sugar is a potent signal for
many developmental processes, including seed germination. The components of the
sucrose non-fermenting (SNF) catabolic repression system (yeast) have been
identified in plants and shown to interact with components of the yeast system in vitro. Rice SNF1 genes are
transcribed during seed development and it is thought that the SNF system is
conserved throughout the eukaryotes and therefore, likely to be functional in
plants, controlling the switch between anabolism and catabolism. There is a
fundamental switch in the primary metabolic processes occurring in seeds between
development and germination. During development, the majority of the incoming
assimilate is put into storage reserves (anabolism) so the seed primarily
synthesizes compounds from smaller molecules. During germination, this process
is reversed where the large storage polymers are catabolized in one part of the
seed and used to make energy and structural molecules in others. The
de-repression of catabolism in the germinating seed is absolutely necessary for
radicle protrusion. Embryos excised from non-germinable barley seeds were still
viable, capable of completing germination and forming autotrophic plants if
they were given a metabolizable carbon source.
An
example of how important the ability to mobilize stored reserves are to the
completion of germination has been provided by the mutant aptly named ‘comatose’. The comatose (cts) mutant is
unresponsive to GA, much like the sleepy
and sneezy mutants mentioned above. The
CTS gene encodes a transporter of
acyl CoA situated in the peroxisome where it orchestrates the transport of acyl
CoAs (produced in the lipid body through breakdown of storage lipid) into the
peroxisome for energy and carbon-skeleton production. In the absence of a
functional transporter, the lipid storing seed (Arabidopsis in this instance) literally
does not have sufficient energy to germinate and hence ‘stalls’ prior to the
completion of germination.
An alternative
role for cell wall hydrolases (as opposed to the breakdown of the endosperm
cell walls to permit the radicle to push through) is in protection of the seed
from pathogen attack. Seed germination takes place in or on soil that is rife
with microbes that can potentially infect and destroy the seed. This presents a
quandary. While the seed is intact, the testa usually provides an effective
barrier to infection. But the embryo itself must penetrate the seed covers from
the inside in order to complete germination thus exposing the internal seed
environment, moist and nutrient rich, to soil microbes. There are mechanisms
that seeds invoke that prevent infection before and after radicle protrusion.
Enzymes with a putative role in protection from infection (pathogenesis
related, PR proteins) have been shown to accumulate in the cells of the
endosperm cap of germinating seeds and some of these PR proteins are cell wall
hydrolases. So, perhaps, cell wall hydrolases are not present in the seed cell
walls to weaken these cell walls so much as they are there to inhibit microbial
attack.
Shen-Miller, J., et al.
1995. Exceptional seed longevity and robust growth: Ancient Sacred Lotus from