PLS 622, Plant Physiology I, Wednesday, October 18, 2006
Reproductive
development:
Objectives for this lecture are to
learn and understand:
-
The stages of pollen development in the anther.
-
The progressive alteration of anther tissues leading to dehiscence.
-
The four main facets of anther dehiscence.
-
Aspects of pollen adherence and rehydration.
-
Pollen tube growth and guidance.
The male
gametes are produced in the anthers,
usually found, in almost all angiosperms, at the end of the filament. The anthers (collectively
known as the androecium) are
comprised of microsporangia (pollen
sacs). The anthers and the filaments together are referred to as the stamens.
In
arabidopsis, there are six stamens, two short and four long, arising from the
floral meristem after the formation of the floral buttress and sepal primordia. These stamen primordia
invaginate towards the base and effectively define the lower portion that will
become the filament from the upper portion that will become the anther. The
filament next elongates at the same rate as the gynoecium so that upon attaining maturity, the anthers are
positioned to dust the stigma with prolific amounts of pollen.
Pollen
development, pollination, and fertilization:
There are
three main phases of pollen development. The first is the development of the
sporophytic cells and meiosis; the second, the formation of free microspores; the third, microspore
mitosis up to and including the formation of the two generative cells and one vegetative
cell.
Stage
I and II:
The sporophytic cells responsible for the
generation of the male gametes divides to produce one tapetal initial and one sporogenous
initial (pollen mother cell). Subsequent sporogenous cell meioses produce a
pollen tetrad of haploid cells
surrounded by a cell wall comprised of callose. This callose cell wall is
degraded by enzymes released from the tapetal layer, primarily callase that is
necessary but not sufficient to release the individual cells of the tetrad from
each other to generate free microspores. At least two other enzymes are
required for tetrad release as evinced by the phenotype of the quartet
mutants qrt1-1, qrt2-1, and qrt3-1. These
mutants produce normal amounts of callase and yet do not separate. Recent
evidence suggests that the quartet
mutants may be/are deficient in pectin degradation. Biochemical evidence
suggests that the qrt1-1 mutant is
deficient in a pectin methylesterase while the qrt2-1 is deficient in a polygalacturonase because the pectin
cementing the tetrad together is not degraded as it is in wild type pollen of Arabidopsis.
Neither QRT1 nor QRT2 are cloned to date but QRT3
has been identified and it is indeed a gene that encodes a protein capable of
pectin degradation, a polygalacturonase.
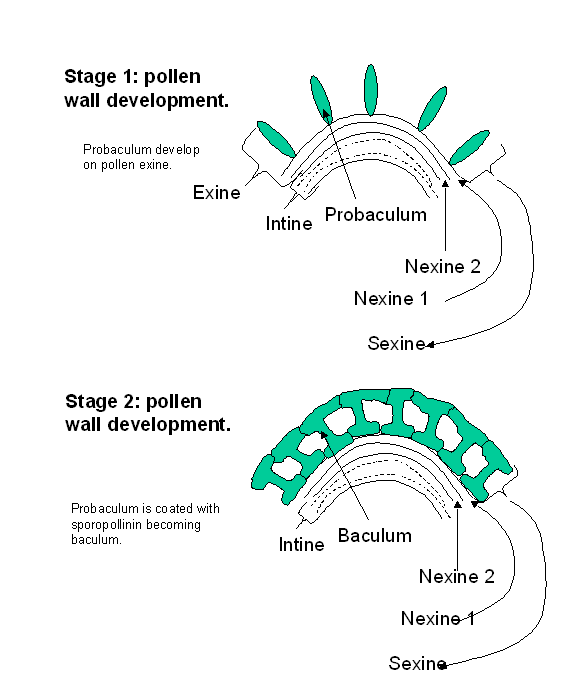
While the
pollen tetrad is still surrounded by the callose wall, each pollen grain is
surrounded by two layers, an inner intine
and an outer exine. The intine is
comprised primarily of pectin and cellulose while the exine is comprised of a
complex, very resilient material, sporopollenin.
The exine is laid down in a species specific pattern in an as yet undetermined
fashion by the sporophyte. Pores develop on the maturing pollen grain where the
exine is reduced or absent leaving only the intine. It is through the pores
that the pollen tube germinates. Upon the generation of free microspores the
rate of deposition of the exine increases and the circumference of the
microspores increases.



Stage
III:
The
uninucleate microspores undergo asymmetric mitotic division, producing a large
vegetative cell and a smaller generative cell enclosed within the vegetative
cell, a so-called bicellular
(previously named binucleate) pollen grain. This division can be considered a determinative division in that the two
cells thus produced undergo very different fates. If the asymmetry of the
mitotic division to produce bicellular pollen is altered, the microspores do
not develop as gametophytes but rather as sporophytes that form a haploid
callus and/or haploid plants. In most plants, the bicellular pollen grain is
released upon dehiscence and the second mitotic division, to produce two
generative cells, occurs after pollen germination as the pollen tube grows
through the style. In some plants however, a third cell, formed from a second
mitotic division of the generative cell, is produced forming a tricellular (formerly trinucleate)
pollen grain prior to anther dehiscence. Typically, these tricellular pollens
are very short lived.
Pollination:
Upon attaining
maturity, the anther bursts apart (dehisces), along a thin region of the anther
where the inner endothecium cells
fail to develop leaving only the epidermis. A specialized cell group referred
to as the stomium (portion of the
anther where the endothecium does not develop, leaving the epidermal cells only)
occupies this position and it is their programmed destruction that permits
normal dehiscence, exposing the mature pollen grains. There are four main
facets to anther dehiscence. These are; 1) the appearance of thickened regions
of fibrous bands of unknown constituents in the endothecial cell wall; 2)
destruction of the circular cell cluster
which permits the joining of the theca
in adjoining anthers; 3) destruction of the connective and tapetum; and 4) the
destruction of the stomium allowing anther dehiscence and the release of the
mature pollen grains. This series of events parallels pollen maturation events
commencing at the time of tetrad formation but it is not dependent on signals
from pollen to progress since male-sterile plants follow the same dehiscence
schedule as normal plants. Depending on the species, the pollen is either
displayed for attachment to visiting insects and/or birds and/or mammals or
released for wind dispersal.
Dehiscence
of the anther is the first stage leading to pollination and is under genetic
control. Numerous mutants have been isolated that do not permit the release of
otherwise viable pollen from the anthers usually due to a defective stomium.
These include the msH mutant in arabidopsis, and ps mutants in tomato.
Pollen
germination:
Upon
landing on a compatible stigma, the pollen must often re-hydrate, the pollen
tube protrude through one of the pores on the pollen exine, extend through the
stylar tissue, locate the ovary and deliver its two generative nuclei to the ovule
completing double fertilization in the angiosperms. In most plants all of the
proteins necessary for the pollen grain to complete germination are already
present in the mature pollen grain when it lands on a stigma. Hence, pollen
germination in most plant species can occur without protein synthesis, although
there are exceptions. Additionally, although pollen grains can be germinated in vitro, this occurs only by mimicking
the chemical environment of the female stigma. Hence, as one plant
developmental geneticist recently put it, the development of the male
gametophyte from pollination to fertilization is dependent on/controlled by the
female reproductive system.
Adhesion
of the pollen grain to the stigma is a controlled event. Pollen grains from
non-crucifer species do not adhere as tightly to the stigmatic surface of
Brassica as do grains from species of the Cruciferae.
The
very act of rehydration is one that is tightly controlled by the maternal plant
tissue. It has been demonstrated that aquaporins, water-conducting channels, in
conjunction with a receptor protein kinase are activated when compatible pollen
lands on a stigma, thereby ensuring that the pollen grain has sufficient water
to re-hydrate and complete germination. Pollen grains from non-compatible
plants are not capable of inducing the aquaporins and therefore remain
dehydrated and do not complete germination. This system, which operates in the
crucifer family, will be discussed in more detail in the next lecture on
self-incompatibility.
Pollen may
need exogenous, diffusible signal molecules, supplied by the stigma, to
complete germination. One of the most convincing arguments supporting this
contention is that mutants in pollen function that do not complete germination
in vitro, can be induced to do so by the addition of wild type pollen, which
presumably supply a signal molecule necessary for pollen germination. In other
mutants deficient in tryphine, a
lipid and protein extracellular coat covering the sporopollenin exine, the
stigma cells in contact with the mutant pollen are induced to form callose
plugs, inhibiting pollen germination.
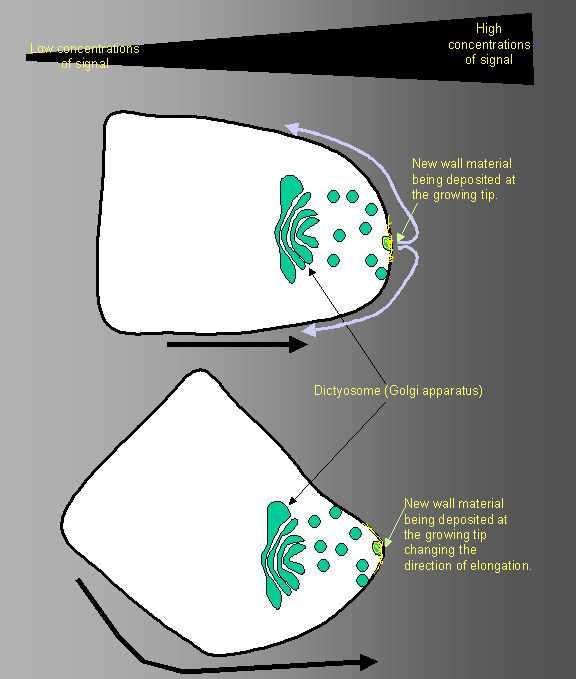
Tip
growth:
Upon
completing germination, the pollen tube elongates through the style toward the
ovary using a unique form of cell elongation (shared by root hairs only)
whereby the components for the tube cell wall are deposited at the end of the
growing tip and which get incorporated and modified as the tip grows beyond
them. This mode of elongation is called tip growth and is in marked contrast to
the usual diffuse elongation found in almost all other plant cells. In tip
growth, the cell wall components required for wall synthesis to permit
continued elongation are deposited at the very tip of the pollen tube by fusion
of dictyosome vesicles with the plasmamembrane. The wall components
subsequently undergo modification such as desterification of pectin as new
waves of wall material deposition displace the former tip wall back along the
tube. Although there is but a single wall at the tip, further back along the
tube there are at least two distinct layers of cell wall.
As the pollen
tube extends into the transmitting style, the cytoplasmic contents become
increasingly diffuse. To maintain appropriate cytoplasmic concentration, the
pollen tube is plugged at intervals with a callose plug. It is important to
note that this is not cytokinesis. Presumably the callose plug allows the
limited cytoplasm to continue to exert positive pressure for tube elongation
within the every increasing length of the tube.
Pollen
tubes are required to make many sharp turns as they grow down the transmitting
tract toward the ovules. Especially when they are approaching the micropyle,
the route out of the transmitting tract, along the funiculus, into the
micropyle is tortuous. Add to this the fact that many tubes are physically
bound to the transmitting tract epidermal cells (see below) and incapable of
altering their position once so bound, and it is easy to see the advantages tip
growth has to offer in getting the tube to the micropyle (see figure on page
9). Should a change of direction be required, the Golgi apparati realign to
commence deposition of wall material on the face of the tube perpendicular to
the preferred direction of growth.
Pollen
tube guidance systems:
The nature of
the chemical thought to provide guidance to the pollen tube has recently been
the topic of much investigation. In tobacco, a transmitting tract-specific glycoprotein (TTS protein, an arabinogalactan protein) was discovered and has
been proven to function at least for pollen adhesion. In lily, a species with a
hollow transmitting tract, two other molecules have been discovered that are
produced by the stigma and style and assist in binding the pollen tube to the
transmitting tract epidermis. These molecules have been identified as a small
cysteine-rich protein similar to lipid transfer proteins and now called the Stigma/Style Cysteine-Rich Adhesin
(SCA). It has a remarkable ability
to bind to pectin and the second molecule produced by the transmitting tract
epidermis was a pectin. Not only can
SCA bind to the stylar pectin, it can also, simultaneously bind to the pectin
present on the pollen tube wall, thereby tightly binding the tube to the
transmitting tract epidermis. In one scenario, a pollen produced
arabinogalactan protein (AGP) similar to the TTS protein discovered in tobacco
(see above), is released by the pollen tube and aids the retention of pollen
tube pectin in the tube wall (see handout figure).
A very elegant
guidance system has been elucidated by Daphne Preuss’s Lab. They found that the
small amino acid gamma amino butyric acid (GABA) is produced in large
quantities throughout the stigma, style, and the integument cells at the
micropylar end of the ovules. There is an enzyme (GABA transaminase) that is
also produced in increasing amounts by these same cells the further away from
the ovule one proceeds. Hence, the integument cells produce a lot of GABA but
little GABA transaminase, so GABA concentration remains high, while the stigma
produces a lot of GABA but also a lot of GABA transaminase which degrades GABA
to Succinic semialdehyde and GABA concentrations are low. Thus, a concentration
gradient of GABA is set up that has the most GABA present in the integuments of
the micropyle and the least present in the stigma. Not only that, but the
pollen tube itself synthesizes the same GABA transaminase. Hence, the GABA that
enters the pollen tube as a signal is rapidly degraded, keeping the pollen tube
responsive to GABA because GABA does not build up in the tube and overwhelm the
sensory mechanism responsive to it. The loss of the GABA transaminase due to
mutations in the gene encoding this protein result in decreased fertility.
These so called POllen-Pistal Incompatible (pop)
mutants produce pollen that are inhibited in tube growth through pop styles
(due to excessively high GABA concentrations) and which cannot ‘find’ the
micropyle again due to overwhelming GABA concentrations at the ovule and the
tubes inability to degrade this GABA.

There are two
basic forms of style in angiosperms. One is a solid stigma and style (such as
in arabidopsis) the other is a hollow stigma and style (as in the lily). In the
former case, the pollen tubes must grow through the transmitting stylar tissue,
through the ovary and find their way to the ovules and through the micropyle.
In the latter case, although the tube need not force its way though tissue it
grows along the inside wall of the style and is guided by molecules comprising
this wall. In either case, directional growth is thought to occur by a
combination of chemotropism and pistil structure. Flavanoids, responsible for
imparting the yellow color to pollen, are one of the chemicals necessary for
normal pollen growth. Mutants deficient in chalcone synthase CHS are normal in
all aspects except that they are white in color and are not capable of
fertilization. In maize, white pollen is produced by plants carrying two
recessive mutations c2 and whp, which both code for chalcone
synthase. Additionally, the flavonids necessary for proper pollen function can
be supplied by either the stigma or the pollen grain itself. It appears that
the flavanol necessary for pollen germination is kaempferol.
Guidance
into the ovule:
There
appears to be considerable evidence that the guidance of the pollen tube to the
micropyle of the embryo sac is based on a chemical signal (GABA is one)! This
chemical signal is sufficient to attract most pollen tubes to the micropyle
from as far away as 75 mm.
Additionally, experiments with arabidopsis have demonstrated that usually only
one pollen tube visits each ovule. Hence the nature of the chemical signal from
the ovules is complex in that is must be sufficient to attract a pollen tube to
achieve fertilization (the raison d’etre for this whole elaborate mechanism)
while being ephemeral so that, once utilized by a pollen tube it quickly
dissipates so that no more pollen tubes are attracted to the now fertilized
zygote. This fits well with what we know of GABA. Once a pollen tube has traversed
through an area, it has absorbed and degraded much of the GABA that had been in
the transmitting tract or coming from the micropyle. This lower GABA amount
will not attract additional pollen tubes which are following greater GABA
concentrations elsewhere in the transmitting tract.