PLS 622, Plant
Physiology I: Monday, September 25, 2006
Vegetative
development: Root hair, and stomatal Development:
Objectives:
- To investigate the
control of root hair development at the morphological and molecular level.
- To determine the molecular control of stomatal spacing and
density.
- To construct an
overview of the roles of the similar molecular players in the various
developmental processes mentioned above.
Root hair development:
Root hairs have been hypothesized to expand the surface area
of a root in order to enhance the water and nutrient uptake efficiency of the root.
They are also the site of attachment of soil mycorhiza. Root hair development
parallels trichome development in many aspects. Like trichomes root hairs arise
from epidermal cells (trichoblasts) that are
over anticlinal cell walls of the underlying cortex.
Microscopically, trichoblasts can be discerned from
atrichoblasts early in their development, prior to the elongation of the root
hair, by having simpler plastids, larger nuclei and nucleoli, greater amounts
of protein, and RNA. This gives the trichoblasts a more cytoplasmically dense
appearance under normal light microscopy indicative of a delayed maturation as
the cell prepares to put forth the cell extension, the root hair. The growth of
the root hair has much in common with the growth of a pollen tube in that it
occurs through the phenomenon of tip growth as distinguished from the diffuse
growth of the vast majority of cells. In tip growth, the cell wall components
required for wall synthesis to permit continued elongation are deposited at the
very tip of the trichoblast by fusion of dictyosome vesicules with the plasmamembrane
at the apex of the trichoblast. The newly deposited wall material is
intercalated into the existing wall and is displaced towards the periphery of
the cell and down along the sides of the cell extension as the trichoblast
increases in length. The wall components subsequently undergo modification such
as desterification of pectin as new waves of wall material deposition displace
the former tip back along the trichoblast.
Root hairs elongate at a defined period of root maturation.
In arabidopsis, the so called "root hair zone" of the elongating root
is located about a millimeter behind the root tip. Cells in this region first
commence putting forth root hairs. Root hairs can eventually attain lengths of
1.5 mm (small in relation to a cotton ovule trichome but still impressive for a
single cell) elongating at a peak rate of up to 100 mm an hour!
Depending on the species, the cells that will produce root
hairs (trichoblasts) are determined by a variety of means. These are summarized
below and in figure 3. Please note that, at the time the fate of root hairs is
being decided, the lateral root cap cells have not yet died and sloughed off
the root epidermis. Hence, the epidermis in the figures is still encased in
lateral root cap cells. This will not be the case later during root development
when the root cap cells have died. This will be an important distinction in a
later lecture when we discuss the differentiation of lateral root cap cells and
epidermal cells that, in Arabidopsis, come from the same initial.
-1) plants in which the pattern of epidermal cell
differentiation (trichoblast or atrichoblast) appears random.
-2) plants in which epidermal cell fate appears to be linked
to asymmetric cell division with the larger daughter cell remaining an atrichoblast
and the smaller developing into a trichoblast. This group includes many
monocots.
-3) plants, including arabidopsis, that dictate epidermal
cells positioned in the crevice between underlying cortex cells (situated over an
anticlinal division of underlying cortical cells) to pursue the trichoblast
pathway, while atrichoblasts form from epidermal cells positioned on top of
cortical cells (situated outside a periclonal division of underlying cortical
cells).

Figure 3:
Determination of root hair development.
In fact, the same molecular players that determine trichome
spacing and development also function to determine root hair fate, although the
logic is reversed. In determining which epidermal cells of the root will be
come root hairs, a similar transcriptional complex to the one we saw in
trichome development decides whether the GL2 gene is up- or down-regulated.
However, now, if GL2 is up-regulated the cell retains the atrichoblast fate,
remaining an undifferentiated root epidermal cell. If GL2 is silenced, the cell
becomes trichoblastic and a root hair develops (Fig. 4). In this instance, the
identity of the bHLH protein(s) remains the same as in the trichome complexes
we have studied above. Both GL3 and/or EGL3 participate in a homo- or
hetero-dimer and bind to TTG1. The MYB transcription factor named WEREWOLF
(WER) associates with the bHLH proteins and TTG1 in the roots to define
epidermal (atrichoblast) fate. CPC (rather than TRY as we saw in the trichomes)
associates with the trichoblast stimulatory complex in the roots (Fig. 4). It
is rather confusing that GL1, which is part of the trichoblast stimulatory
complex in trichome formation, is replaced in the root epidermal cells by a
functionally identical MYB transcription factor, WER that now defines the
trichoblast inhibitory complex. The story is even more complex because, the
coding regions of GL1 and WER can be swapped and GL1 can be placed under the control of
the WER promoter so it is only
expressed in the roots. Conversely, WER
can be placed under the control of the GL1
promoter so it is only expressed in the shoot. Despite the fact that the WER
protein in the root directs the atrichoblast fate, in the shoot it can direct
the trichoblast fate! The converse is true of GL1 in the root! So, the only
determinant directing whether WER is stimulatory (shoot) or inhibitory (root)
for trichoblast fate is where the gene is transcribed (positional
determination). The same is true for GL1.
Of the three
models in figure 3, the third, describing Arabidopsis root hair development, is
depicted in figure 4. Some as yet unidentified, GL2 transcription stimulatory substance is thought to pass from the
underlying cortical cells into the overlying epidermal cells (red arrows). At
the edges of the cortical cells the apoplast between adjoining cells results in
a region incapable of generating this message and transporting it into the
overlying epidermis. Hence, epidermal cells positioned over a cell-cell
junction in the cortical cells, receives less of this stimulatory substance,
setting that cell up to become trichoblastic (Fig. 4).
Stomatal development:
Stomata are essential to the life of terrestrial plants.
There is usually a stomata free zone surrounding each stoma in normal plants. This
intervention of subsidiary (when they exist) or epidermal cells between stomata
is thought to provide a pool of ions for the guard cells
and optimize the access of the underlying mesophyll cells to
the external environment. Two key features combine to influence stomatal patterning;
internal anatomy (cell type, position of the vasculature), and contact between
stomata. Little is known respecting why stomata only rarely develop over the
vasculature. Even less is known about how certain underlying cell types seem to
inhibit stomata from developing over them. Hence, we will discuss the control
of stomatal patterning such that stoma rarely develop side-by-side.
Stomatal initials form by asymmetric cell division of
precursor cells such that the smaller cell becomes a stomatal precursor. The
stomatal initial can produce both the guard cells and ordinary epidermal cells.
Considerable cell-to-cell communication takes place leading to the coordination
of the plane of cell division as well as the regulation of precursor cell
activity. Additionally, the timing of when a cell is competent to become a
stomatal precursor and where such precursors can initiate are highly
controlled.
In both dicots and monocots, the cell divisions leading to
the production of stomata are highly asymmetric both in geometry and in the fate
of the cells thus produced. In monocots, the first asymmetric division results
in the smaller, usually rectangular cell becoming a guard mother cell which
divides symmetrically producing two guard cells. In

dicots, the smaller cell is usually triangular and continues
to divide after the surrounding cells have ceased to do so. Eventually, it converts
into a guard mother cell and divides symmetrically to produce two guard cells
(Fig. 4). Since dicot stomatal initials continue to divide after the
surrounding epidermal cells have ceased they have been termed meristemoid cells
to emphasize their ability to continue cell division. In dicots there are two
types, primary meristemoids and secondary (satellite) meristemoids, the latter
produced by an asymmetric division of a neighboring cell to the stomata.
Rectangular initials (a.k.a. short cells) in monocots
comprise the three types of stomatal initial found in the angiosperms. How
stomatal pattern is initiated depends on the type of initial.
Primary meristemoid positioning appears to be random, with
the exception that it obeys the rule that two stomata should not be adjacent. Secondary
meristemoids and stomatal initials in monocots are placed in a highly regular
fashion crucial to stomatal patterning. The asymmetric division producing the
stomatal initial in monocots occurs so that the initial is placed distal from
the base of the leaf. Upon the formation of the initial, asymmetric divisions
occur in cells adjacent to the new initial to form two subsidiary cells.
Finally, the guard mother cell divides symmetrically to form two guard cells
surrounding a pore (Fig. 5).
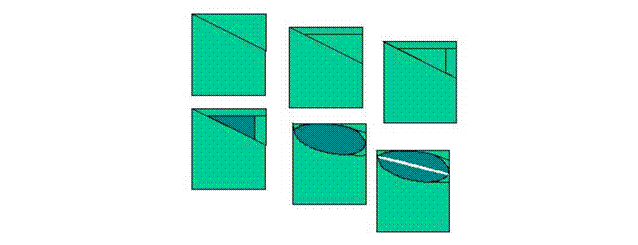
Figure 4: Dicot
stomatal development.
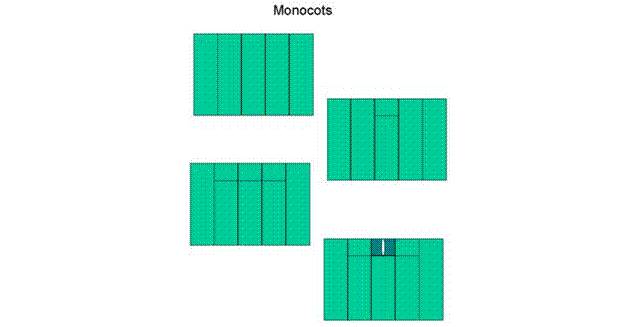
Figure 5: Monocot stomatal development.
Primary meristemoids arise from protodermal cells. These
cell undergo a series of asymmetric divisions that produces a small, triangular
initial situated approximately in the middle of the future stomatal complex.
The meristemoid next alters shape to an oval guard mother cell. Finally, as in
monocots, the mother cell divides symmetrically and produces two guard cells.
Satellite meristemoids arise once a primary meristemoid has
completed development into a stoma. One of the surrounding cells maintains meristemoid
identity and undergoes asymmetric division such that the guard mother cell is
formed distal from the existing stoma. Upon several asymmetric divisions, the
meristemoid, now separated from the existing guard cells by epidermal cells,
also converts to an oval guard mother cell and divides symmetrically.
My question to you is: what would be the outcome of
conducting an experiment like the one with the transposon interrupted GUS gene
used to elucidate trichome developmental patterns (Fig. 1, above) if applied to
stomatal development?
Timing of stomatal
development:
In arabidopsis at least, stomata develop after trichomes
have formed.
Cell-to-cell
communication during stomatal development:
There is a wide array of opportunities for signals to be
traversing between cells thus coordinating stomatal development. Evidence for cell-to-cell
communication include the inhibition of stomata to form above veins in the
leaf, the formation of satellite meristemoids
situated away from existing stomata, the arrested
development of meristemoids located proximally to each other or to stomata, the
orientation of subsequent cell divisions to separate meristemoids when two do
happened to develop beside each other and, the
initiation of subsidiary cell formation in monocots.
A genetic analysis of
stomatal patterning:
There are two mutants identified that violate the rule that
no two stomates should be in contact. However, the four lips (flp) and too many
mouths (tmm) mutations transgress by two different developmental mechanisms.
tmm mutants have far greater numbers of stoma in rosette leaves, cotyledons,
and abaxial sepal surface. Other tissues of tmm plants (inflorescence stem,
adaxial sepal epidermis, silique tips) entirely lack stomata whereas wild type
plants possess them. The flower pedicle shows an abnormal gradation of stomatal
frequency from none proximal to the stem to abnormally greater numbers near the
flower base. Hence, TMM is thought to control the entry of protodermal cells
into the stomatal developmental process and appears to have opposite effects on
meristemoids depending on the tissue (compare rosette leaves with sepal epidermis).The
tmm mutation appears to increase the formation of satellite meristemoids as
well as to alter the polarity of cell divisions such that meristemoids can now
form next to guard cells.
The flp mutation can produce unpaired guard cells as well as
result in stomatal twinning. Unlike tmm mutants, flp mutants do not exhibit increased
numbers of guard cells. Analysis of stomatal development in flp mutants
suggests that stomata develop normally until the guard mother cell stage. At
this stage the guard mother cell undergoes a symmetric division and the
resulting daughter cells divide symmetrically again to produce two, contiguous
stomates. Hence FLP may control guard mother cell identity, or regulate the
number of symmetric divisions a guard mother cell can undergo.
The R-558 mutation causes an increase in stomatal density
throughout the plant and stomatal clustering can also occur.
References used in the preparation of these notes.
Costa S, Dolan L. 2003. Epidermal patterning genes are
active during embryogenesis in Arabidopsis. Development 130, 2893-2901.
Koshino-Kimura Y, Wada T, Tachibana T, Tsugeki R, Ishiguro S
and Okada K. 2005.
Regulation of CAPRICE Transcription by MYB Proteins for Root
Epidermis
Differentiation in Arabidopsis. Plant Cell Physiol. 46: 817–826.
Nadeau JA, Sack FD. 2002. Control of stomatal distribution
on the Arabidopsis leaf surface. Science. 296: 1697-1700.