
The Chemical Defenses of Higher
Plants
Even
a casual consideration of the relation between higher plants and the multitude
of animals that consume them makes one marvel at the plants' ability to
survive. When it is attacked by a predator such as an insect, a plant can
neither run away nor defend itself physically. Yet many plants have evolved
subtle ways to protect themselves that are no less effective: they employ
chemical defenses. Such defenses may be simple or elaborate. Some plants
manufacture toxins that poison the attacking herbivore, whereas others
produce complex compounds that interfere with the attacker's growth cycle
or its ability to digest the plant.
Insects and other herbivores have in turn developed responses to this chemical warfare. Many herbivores have managed to adapt to the defensive mechanisms of plants, developing chemical defenses of their own. Some insects have developed ways to convert potentially harmful substances produced by plants into sources of nutrition or protection from insectivores. The study of such chemical interactions among organisms forms the basis for an emerging discipline known as chemical ecology.
The chemical ecologist studies the role of natural chemical products in the relations among organisms. One such relation is feeding: all plant-eating insects have basically the same nutritional requirements, which can be satisfied, more or less, by most higher plants. What is it, then, that determines an insect's specific feeding pattern? On what basis does it select or reject a particular plant as a food resource?
As the late Gottfried S. Fraenkel suggested, it is not a plant's primary metabolites (the substances it synthesizes that are essential for its growth and reproduction) that make it suitable or unsuitable as a source of food. Rather, the plant's suitability depends to a large degree on its secondary metabolites: metabolic compounds that are not involved in the common processes of life and that vary from plant to plant, helping to determine each plant's unique characteristics.
In 1971 the late Robert H. Whittaker and Paul P. Feeny of Cornell University added a new level of precision to Fraenkel's concept. They suggested that secondary metabolites produced by an individual of one species and able to affect the growth, health, population biology or behavior of another species should be called allelochemics. (Chemical ecologists now use the terms allelochemic and allelochemical interchangeably; I much prefer the latter term.) Among the many types of allelochemicals are attractants, repellents, allergenics and toxins. In this presentation, I shall discuss the allelochemicals employed by certain plants to defend themselves from predation by insects and various other herbivores.
The customary way to determine the defensive capacity of a higher plant's allelochemicals is to demonstrate their toxicity toward one or more of a variety of insects that have come to be accepted as standard reference species in evaluating biological toxicity. The usual approach is to incorporate the natural allelochemicals into an artificial diet that would normally sustain the insect. J. M. Erickson, then a student of Feeny's, and Feeny, working on the black swallowtail butterfly, Papilio polyxenes, modified this method to provide a more natural approach. Instead of creating an artificial diet for their insects, they introduced a plant allelochemical into a plant that is part of the butterflies' natural diet.
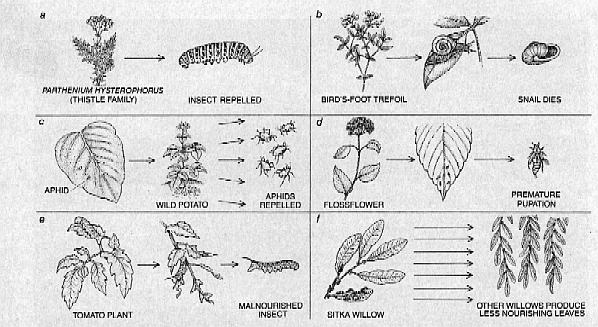
Adult P. polyxenes avoid plants of the group Cruciferae (the mustards) which produce such allelochemicals as sinigrin, a compound that contains allylisothiocyanate, a toxic constituent. On the other hand, the butterflies forage avidly among the Umbelliferae, which include such plants as celery. Erickson and Feeny reared P. polyxenes larvae on a diet of celery leaves that had been induced to take up sinigrin. The larvae fed, and their growth was markedly inhibited. Celery containing a level of sinigrin equivalent to the level found in cruciferous vegetation was lethal to all the tested larvae. These experiments demonstrated that toxic allelochemicals could render an otherwise suitable host plant unacceptable to an insect pest.
David A. Jones and his colleagues at the University of Hull developed another experimental verification of the effectiveness of toxic allelochemicals. They studied bird's-foot trefoil (Lotus) and white clover (Trifolium), species that are capable of producing cyanogenic glycosides, compounds made of sugars bound to cyanide complexes, and storing them in their leaves. If two particular enzymes are present when the plant's leaves are damaged, the cyanogenic glycosides are broken down to release the cyanide complex, from which free cyanide is eventually liberated. Bird's-foot trefoil and white clover are "polymorphic" for cyanogenesis: only some individual plants can produce both the cyanogenic glycosides and the enzymes required to liberate cyanide. Hence not all plants of each species can defend themselves by means of cyanogenic glycosides. Jones exploited this peculiar property to determine how effective a stratagem cyanogenesis is for the plant population within his region of study. He examined a map, published in 1954 by Hunor Daday, then at the Welsh Plant Breeding Station in Aberystwyth, showing the geographic distribution of plants able to synthesize both cyanogenic glycosides and the appropriate enzymes and thus able to produce free cyanide.

Daday had found a dramatic relation between the January mean temperature of the regions within his study area and the proportion of clover plants in those regions that could produce free cyanide. In warmer regions, such as the area around the Mediterranean Sea, about 70 to 90 percent of the collected plants exhibited cyanogenesis, whereas in colder regions, such as portions of the U.S.S.R almost none of the analyzed clover was cyanogenic. Jones and his colleagues observed that slugs and snails, two major predators of the bird's-foot trefoil, tend to consume acyanogenic plants rather than cyanogenic ones. The ability to produce free cyanide would therefore be more advantageous to plants in warmer habitats, where slugs and snails remain active during the winter Cyanogenesis would be less valuable to plants in areas where winter weather controls herbivore populations
Another kind of defense employing toxic allelochemicals has been described by Eloy Rodriguez of the University of California at Irvine. In a noteworthy study of the vegetation of Baja California and Chihuahua he has found that the trichomes, or hairs, of many desert plants are storehouses of toxic natural products. The trichomes of the desert plant, Phacelia, for example, contain numerous poisons, insecticides and allergenic substances. The trichomes of another desert plant, the thistle Parthenium hysterophorus, contain certain allergenic chemicals that deter herbivores from feeding.
Some allelochemicals protect plants that produce them not by poisoning or repelling herbivores but by in-terfering with the predators' normal cycles of growth and development. Many insects grow in three morphologically distinct stages: larval, pupal and adult. Some go through several larval stages before pupating. Certain other insects do not go through morphologically distinct stages; the hatchlings, which resemble adults in appearance, do go through several periods of ecdysis, or molting, however, growing in size at each stage. Two types of hormones that play a key role in insect development are the juvenile hormones produced by larvae and a hormone group known collectively as ecdysteroids, which act to initiate the cycles of ecdysis that occur within the developmental sequence. Adolf F. J. Butenandt of the Max Planck Institute for Biochemistry in Munich and Peter Karlson, who was then at the University of Tubingen, were the first investigators to isolate ecdysteroids. From nearly 1,000 kilograms of silkworms they eventually isolated 25 milligrams of an ecdysteroid called ecdysone and about a third of a milligram of another one called 20-hydroxyecdysone. Soon afterward Koji Nakanishi, now at Columbia University, and T. Takemoto of the University of Tokushima discovered that certain plants are excellent sources of ecdysone-like materials. They were able to extract 25 milligrams of 20-hydroxyocdysone from a mere 2.5 grams of a dried rhizome (a subterranean food-storage organ) of the common fern, Polypodium vulgare. That inspired a search by many other investigators for other phytoecdysones (plant-produced ecdysteroids). Eventually several dozen structurally different compounds were isolated, a few of which were found to be even more potent than similar compounds that occur naturally in insects.
In recent years Isao Kubo and his colleagues at the University of California at Berkeley, working in Kenya, confirmed that phytoecdysones can act as powerful protective agents by disturbing the growth cycles of insects that prey on phytoecdysone-producing plants. These workers found that after swarms of locusts had virtually denuded broad stretches of savanna vegetation the sole surviving plant species was a bugleweed, Ajuga remora. When they fed extracts of A. remora to a number of insects, a striking developmental aberration resulted: in metamorphosing from larvae to pupae, each insect grew not one but several head capsules.

The insects" extra head capsules blocked their mouthparts and they starved. Kubo established that this developmental abnormality was due to the presence in the plant of several phytoecdysteroids that had blocked the normal metamorphosis from larva to pupa. In each insect the cellular events that normally initiate metamorphosis had occurred but the actual shedding of the larval exoskeleton had been aborted. Just as A. remora protects itself by producing analogues of ecdysteroids, so certain other plants employ analogues of juvenile hormones. In many insects juvenile hormone acts as a control on the process of development. As long as the growing larva produces juvenile hormone, ecdysteroids can initiate a molt only from one larval stage to the next, the insect cannot metamorphose into a pupa unless the juvenile hormone is degraded and thus unable to influence the process of pupation directed by ecdysteroids. Juvenoids, which are substances that re-semble juvenile hormones but are pro-duced by plants, can be of significant protective value to the plant if they are lethal. (Otherwise they may only prolong the insects' larval stage, which, ironically, is typically the most destructive phase of an insect's life.)
The first juvenoids were discovered in 1964, when Karel Slama came from the Entomological Institute of the Czechoslovak Academy of Sciences to work with Carroll M. Williams at Harvard University. The two investigators planned collaborative studies of Pyrrhocoris apterus, a bug found in Europe. P. apterus normally goes through five distinct stages before metamorphosing into an adult. Slama and Williams made the surprising discovery that insects reared at Harvard did not ecdyse from the fifth stage to the adult, instead they went through a sixth and sometimes even a seventh stage. Slama had never observed such a growth pattern in Czechoslovakia. Eventually it became evident that the insects must have been exposed to a juvenile hormone, which was interfering with normal development. A series of tests revealed the critical factor to be the paper placed in the petri dishes that housed the bugs. The investigators tested a variety of paper products and found that many American newspapers and journals caused the same anomalous effect, whereas similar paper products from European and Japanese sources did not. (In Czechoslovakia, Slama had naturally lined his dishes with European-made filter paper.) Slama and Williams were able to isolate the active factor from paper towels and to determine that it was effective only against the Pyrrhocoridae family. A closely related group of bugs, the Lygaeidae, was totally unaffected by the factor. Finally the active factor was traced to the wood pulp from which the paper had been made. Pulp derived from the balsam fir, Abies balsamea, a primary source of pulp for North American paper products, was found to be particularly active.
Several years later William S. Bowers, then working at the New York State Agricultural Experimental Station, isolated and characterized the active factor, which he named juvabione. It is quite similar in structure to insect juvenile hormones. A few years afterward, Bowers decided to determine whether other plants protect themselves by blocking, rather than imitating, an insect's normal juvenile hormone. A juvenile-hormone antagonist could kill insect larvae by causing them to molt prematurely into the adult stage. Eventually he succeeded in isolating two such substances from Ageratum houstonianum, a small plant that grows in temperate regions. He named the two compounds prococene I and prococene II because of their ability to elicit precocious metamorphosis by preventing the secretion of juvenile hormone. Prococene II has a number of other effects as well: it terminates the production of sex attractants by the American cockroach, causes several types of insects to lay infertile eggs and forces the Colorado potato beetle to enter a hibernational state called diapause. When insects that have been fed prococene II are later treated with juvenile hormone, the deleterious effects of the prococene are reversed.
Plants can mimic many other sub-stances that are naturally secreted by insects. For example, the aphid Myzus persicae, which preys on such plants as the wild potato, secretes a substance called an alarm pheromone when it is attacked by a predator. The vola-tile pheromone alerts other aphids to impending danger. An important in-gredient of the aphid's alarm pheromone is a compound called (E)-beta-farnesene. Richard Gibson and John A. Pickett of the Rothamstead Experimental Station in England have found that Solanum berthaulthii, a wild, tuber-bearing potato, releases (E)-beta-farnesene from trichomes on its leaves. Thus the plant can repel a major pest by mimicking that pest's alarm signal. The insect cannot readily counter or adapt to this type of defense, since it cannot ignore the very chemical signal that is critical to its survival.
Some insects have, however, been able to adapt to the chemical defenses of plants. In many cases adap-tation has taken a remarkable turn: sometimes the insect can store and make effective use of the chemical the plant employs as a defense. One example of such an insect is the grasshopper Poekilocerus bufonius, which feeds solely on plants of the Asclepiadaceae, or milkweed, family. The milkweeds manufacture a number of complex compounds known as cardenolides, toxins that can severely disrupt normal cardiac function. When this grasshopper is attacked by a potential predator, it can defend itself by ejecting a spray from a poison gland. Analysis of this fluid reveals that it contains two major cardenolides, calactin and calotropin, both of which can also be extracted from the milkweeds on which the grasshopper feeds. When a grasshopper is maintained on a diet that includes no milkweeds, the cardenolide content of its protective spray is reduced tenfold.

It seems evident that the plant itself is the source of the defensive compounds in the insect's defensive fluid. The existence of insects, such as the grasshopper, that are able to store and utilize a plant's defensive chemicals led Whittaker and Feeny to refine the concept of an allelochemical by dividing the allelochemicals into several groups. Two groups are of particular importance: allomones, which confer an adaptive advantage on the organism that produces them, and kairomones, which confer an advantage on the organism that receives or consumes them. In the case of the grasshopper and the milkweeds, a chemical that may originally have been an allomone (the cardenolide) has become a functional kairomone, benefiting the insect that consumes the plant. The grasshopper's adaptation to the milkweeds' defensive chemicals may be one example of coevolution. Coevolution is a reciprocal process in which the properties and characteristics of one organism evolve in response to specific properties of another organism; the two interacting species exert pressures that influence each other's genome. Some coevolutionary relations between plants and insects have become remarkably specialized. For example, certain flowers can be pollinated only by a single species of insect; the insects in turn exhibit absolute fidelity to flowers of their host plant. There is considerable interest in re-constructing the extent to which higher plants and their insect pests and predators may have evolved together over evolutionary time. In the case of defensive chemicals, perhaps higher plants evolved toxic natural products as part of their defensive barrier against herbivores. Certain herbivores may simply have avoided the plants, whereas others may have evolved an ability to detoxify the plants' defensive chemicals. Perhaps plants counteradapted by intensifying the efficacy of their al-lelochemicals, and perhaps certain insects evolved to the point where the plants' allomones effectively became kairomones.
A nother example of an insect that exploits a plant's defensive chemical as a kairomone is the monarch butterfly. In 1967 Lincoln Pierson Brower, now at the University of Florida, observed that monarch butterflies grown from larvae that had fed on Asclepias curassavica, a milkweed that stores a good deal of cardenolides, were un-acceptable as food for blue jays. Jays that consumed the butterflies, or even certain parts of one butterfly, became violently sick. grower's finding was consistent with observations made by other investigators that butterflies of the sub-family Danainae, which inchldes the monarch, are rejected on sight by a large number of insectivorous birds. Brower analyzed the compounds in adult and pupal monarchs and found about 10 different cardenolides; the total amount of toxin in the body of one butterfly is several times higher than the amount necessary to kill a cat or a small dog. The insects rarely store all the cardenolides found in the host plant, but all the cardenolides the insect does store have a counterpart in the plant. Butterflies grown from larvae maintained on a cardenolide-free diet had no harmful effect on blue jays.
The monarch butterfly is aposematic: it "advertises" its intrinsic toxicity by the bright coloration and distinctive markings of its wings. The presence of such aposematic insects as the mon-arch butterfly has spawned an array of mimics. These mimetic insects, such as the viceroy butterfly, do not store cardenolides, but they resemble insects that do. Thus they are placed under the cardenolide umbrella of protection without the need to produce, regulate, store or utilize the toxic chemicals. Another interesting dimension has been added to investigators' knowledge of these interactions by grower's field work in central Mexico, the wintering ground for a vast mlmber of monarch butterflies. In this habitat, assaults by black-backed orioles and black-headed grosbeaks account for more than 60 percent of the butterflies' mortality. Detailed observation of the feeding activity of these insectivores revealed that the two species of birds feed quite differently. The orioles, which are sensitive to cardenolides, pick the butterflies apart; they selectively remove the thoracic muscles and abdominal contents without eating the cardenolide-laden cuticle (the outer covering of the body) or wings. Grosbeaks, on the other hand, are much less sensitive to the toxins, and they feed randomly, voraciously eating the intact abdomen. No defense is inviolate, and, like the milkweeds, monarch butterflies are not completely protected by their chemical defenses.
There is yet another way in which insects can exploit a plant's chemical defenses. Vincent G. Dethier of the University of Massachusetts at Amherst and Louis Schoonhoven of the University of Wageningen in the Netherlands, along with other investiga-tors, has found that many insects are equipped with taste receptors sensitive to the allelochemicals of plants on which they feed. For example, larvae that consume plants of the family Rosaceae have receptors for sorbitol, a sugar alcohol that is stored by such plants. Other insects usually lack such receptors. Insects equipped with these receptors can rely on their olfactry ability in finding and identifying chemically defended plants to which they are immune or resistant.
So far I have discussed mainly the alleloehemieals that are constitutive to a plant, that is, produced whether or not the plant is under attack by a predator. Many plants rely instead on so-called inducible defenses: protective compounds that are synthesized only in response to an attack. An excellent example comes from the work of Clarence A. Ryan and his colleagues at Washington State University. They have found that when some plants, such as the tomato, are attacked by chewing insects, they release a substanee that travels from the site of the wound through the plant and initiates the production of at least two kinds of macromolec ule called proteinase inhibitors. Proteinase inhibitors impede the insects' ability to break down proteins they have ingested from the leaf. The leaf thus makes itself a less acceptable source of food. The study of inducible defense was recently taken a dramatic step further by the work of David F. Rhoades of the University of Washington, as well as that of Jack C. Schultz and Ian Balwin, then at Dartmouth College. It has been established that when the Sitka willow, Salix sitchensis, is attacked by insects, its leaf quality (a measure of its suitability as a food resource for insects) deteriorates. Rhoades noted, however, that the leaf quality of near-by willows-even willows that had not been attacked-also seemed to deteriorate. Perhaps, Rhoades suggested, the attacked tree produces a signal, analogous to an insect's alarm pheromone, that travels through the air to induce defensive responses in neighboring, unattached trees. Schultz and Balwin tested Rhoades's hypothesis by planting seedlings of the sugar maple, Acer saccharum, in two separate growth chambers. They found that plants that had been damaged intentionally, as well as plants in the same growth chamber as the damaged ones, tended to produce greater quantities of tannins and phenolics, two defensive compounds, than plants grown in a separate chamber. Although these studies have not definitively proved the existence of communicative chemical defense by plants, they have generated great interest in at-tempts to prove that pheromonal com-munication between trees may actually take place.
The chemical and biochemical study of the protective allelochemicals of higher plants can make important contributions to future efforts to con-trol depredations on crops by insect pests. A recent report by the National Academy of Sciences emphasized the rapidly growing number of insects that have developed resistance to current-ly available chemical agents. Biological and chemical studies, the stuff of chemical ecology, may lead to effective pesticides that are less hazardous to the environment and not as easily circumvented. Natural products possess the advantage of structures that have amply proved effectiveness. They also offer excellent opportunities to develop experimental systems for probing the capacity of insects to cope with toxic compounds and may thereby make it possible to undermine that capacity. Natural products may also make it possible to develop novel, nonpesticide approaches to the con-trol of herbivores. It should be possible to exploit natural compounds that deter feeding or the deposition of eggs, or even to grow crops from which the natural substances that attract herbivores or stimulate their feeding behavior have been eliminated.